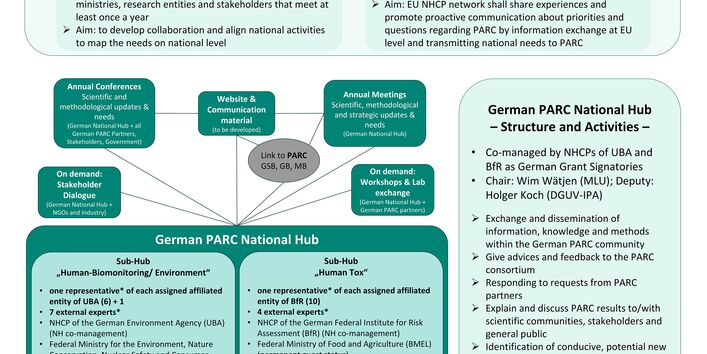
Framework of the Partnership
In May 2022, the partnership "European Partnership for the Assessment of the Risk from Chemicals" (PARC) was launched, within the EU Horizon Europe programme. The intention of PARC is to serve as a pan-European partnership to support European and national risk assessment and risk management authorities in the field of chemicals assessment. The partnership will span seven years (May 2022 to April 2029) with a total budget of €400 million and is supported by the participating member states or their national partners through a financial contribution of 50 percent. The 27 EU member states, as well as the United Kingdom and Switzerland, are involved in the partnership with varying levels of funding, whereby Germany and France are providing the largest financial contributions. The overall coordination of the PARC partnership has been taken on by France through the French Agency for Food, Environment and Occupational Safety (Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail, ANSES). Each participating country is represented by corresponding national governmental authorities, as grant signatories (GS), which for Germany are the German Environment Agency (UBA) and the German Federal Institute for Risk Assessment (BfR). Affiliated entities (AE), in the form of additional national partners, are then in turn connected to each GS. The UBA has six such AE and BfR ten (see section "PARC - National Hub -> Affiliated entities "). Furthermore, the European Environment Agency (EEA), the European Food Safety Authority (EFSA) and the European Chemicals Agency (ECHA) are participating. Finally, five European Directorates General (DGs) of the European Commission provide technical guidance to the partnership: Directorate General for Research and Innovation (DG R&I); Directorate General for Environment (DG ENV); Directorate General for Health and Food Safety (DG SANTE); Directorate General for Internal Market, Industry and Entrepreneurship (DG GROW); and the Joint Research Center (JRC).
Organisational Framework of the Partnership
PARC builds on the work done in HBM4EU, under the European Joint Programme, which was coordinated and led by the Department of Toxicology, Health-Related Environmental Monitoring at UBA (Kolossa-Gehring et al. 2023)2. PARC aims to further this work, amongst other, in particular on an EU-wide human biomonitoring system.
In order to fulfil the set goals and meet the research and innovation needs, PARC is divided into nine content-related work packages (WP). These WP cover a broad range of topics on research and methods while considering aspects of sustainability, as well as innovation and integration, and are supplemented by WP for coordination and steering. For the overall steering of PARC, various decision-making bodies have been established in which e. g. representatives of the national ministries of the participating member states partake. For Germany, representatives of the Federal Ministry for the Environment, Nature Conservation, Nuclear Safety and Consumer Protection (BMUV) and the Federal Ministry of Food and Agriculture (BMEL) participate in these steering bodies.
Content Framework of the Partnership
UBA is represented in eight of the nine WP with a total of 36 employees. Furthermore, UBA has taken on the co-leadership of WP 4 ("Monitoring and Exposure") whereas BfR, as the second GS in Germany, has taken on the co-leadership of WP 5 ("Hazard Assessment"), among other. In WP 4, the exposure of humans and the environment to chemicals will be considered jointly. The studies are focused on the "one-substance-one-assessment approach" (van Dijk et al, 2021)3 with the aim to strengthen the linkage between human health and the environmental data, enabling an integrated assessment. In addition, new methods will be developed and tested in PARC with the goal, inter alia, to improve exposure assessment of particularly vulnerable populations. In WP 4, method development is focused on so-called "screening-methods", which should enable the simultaneous determination of the presence of a large number of chemicals in the environment and in humans. For this purpose, existing monitoring programmes will be further developed and the monitoring results should be systematically used for future authorization of hazardous substances.
2 HBM4EU from the Coordinator's perspective: lessons learnt from managing a large-scale EU project
3 Towards ‘one substance – one assessment’: An analysis of EU chemical registration and aquatic risk assessment frameworks